Key Pain Conditions
Many chronic conditions are challenging to manage. Here we focus on key pain conditions with a high unmet need among patients and/or healthcare professionals.
Our aim here is to provide the pain community with in-depth information about these conditions; from disease understanding to management approaches.
Cancer-related neurotic pain
Cancer-related pain can be caused by “the tumour itself or its metastases inflaming or eroding bone, viscera or nerves, or pain related to tissue or nerve damage induced by cancer treatments”.1 Chronic cancer pain can be categorised as visceral, bone or neuropathic cancer pain.1
Cancer-related neuropathic pain (CRNP) is a result of direct damage to the central or peripheral nervous system from a primary tumour or metastases, or from cancer treatment such as chemotherapy (painful chemotherapy-induced peripheral neuropathy [CIPN]), surgery (post-surgical neuropathic pain [PSNP]), radiotherapy and immunotherapy.1–3 CRNP can lead to cancer therapy dose reduction or cessation, which can increase cancer-related morbidity and mortality.4–6
Quick facts
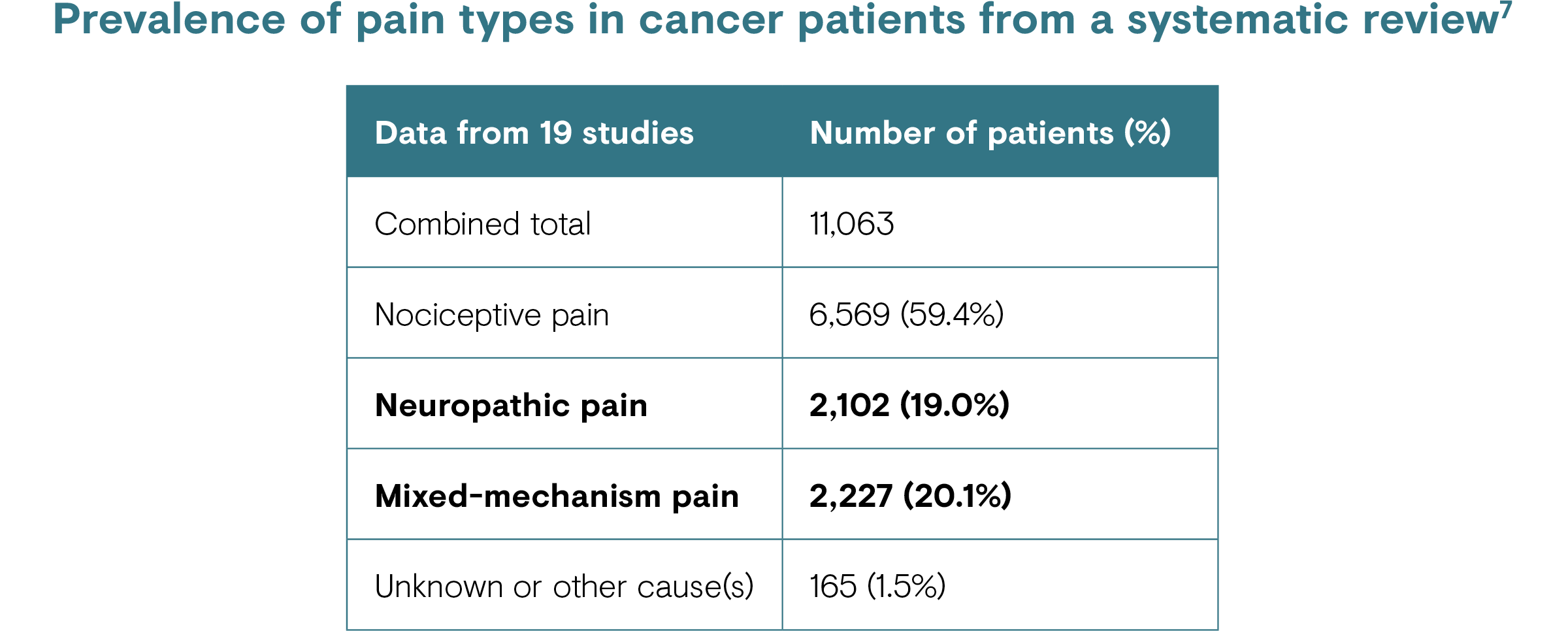
- CRNP is estimated to affect up to 39% of patients with cancer.7
- Causes of CRNP include the tumour and/or its metastases, or pain related to nerve damage induced by cancer treatments.1
- CIPN is a common treatment-related peripheral neuropathic pain (PNP)
and is prevalent in up to 70% of patients after the first month of chemotherapy.4 - Other types of treatment-related PNP are radiotherapy-induced neuropathic pain and PSNP.1
- Neuropathic pain-specific assessment tools, such as the Leeds Assessment of Neuropathic Symptoms and Signs (LANSS) pain scale, the Douleur Neuropathique en 4 Questions (DN4) questionnaire and the painDETECT questionnaire (PDQ) have been evaluated for use in CRNP.8–14
- Pharmacological management guidelines for cancer pain have been published by the European Society for Medical Oncology (ESMO),15 the American Society of Clinical Oncology (ASCO),16 the European Pain Federation (EFIC)17 and the National Comprehensive Cancer Network (NCCN).18
- ESMO, European Oncology Nursing Society (EONS) and the European Association of Neuro-Oncology (EANO) have also published guidelines for diagnosis, prevention, treatment and follow-up of systemic anticancer therapy-induced peripheral and central neurotoxicity.19
Epidemiology
Worldwide, it is estimated that there will be 27.5 million new cases of cancer each year by 2040.20 A 2016 systematic review of prevalence of cancer-related pain in an adult population reported that 66% of patients with advanced, metastatic or terminal disease will experience pain, and that 38% of all patients with cancer will experience pain of moderate to severe intensity.21
CRNP is estimated to affect up to 39% of patients with cancer, when considering both pure neuropathic and mixed (e.g. neuropathic and nociceptive) pain.7 CIPN, a common treatment-related CRNP/PNP, has been reported to be prevalent in up to 70% of patients after the first month of chemotherapy, 60% at 3 months and 30% at 6 months or more.4
Risk factors
Factors that increase the risk of CRNP range from the type of cancer, its stage (i.e. people with advanced cancer are more likely to have pain), the type and dosage of treatment(s) or therapy used [see the ‘Causes’ section], and comorbidities (e.g. pre-existing neuropathy).1,6,22,23 It is estimated that two-thirds of neuropathic pain in patients with cancer is tumour-related: around 20% results from cancer treatment and 10–15% is from comorbid diseases. 7

Causes
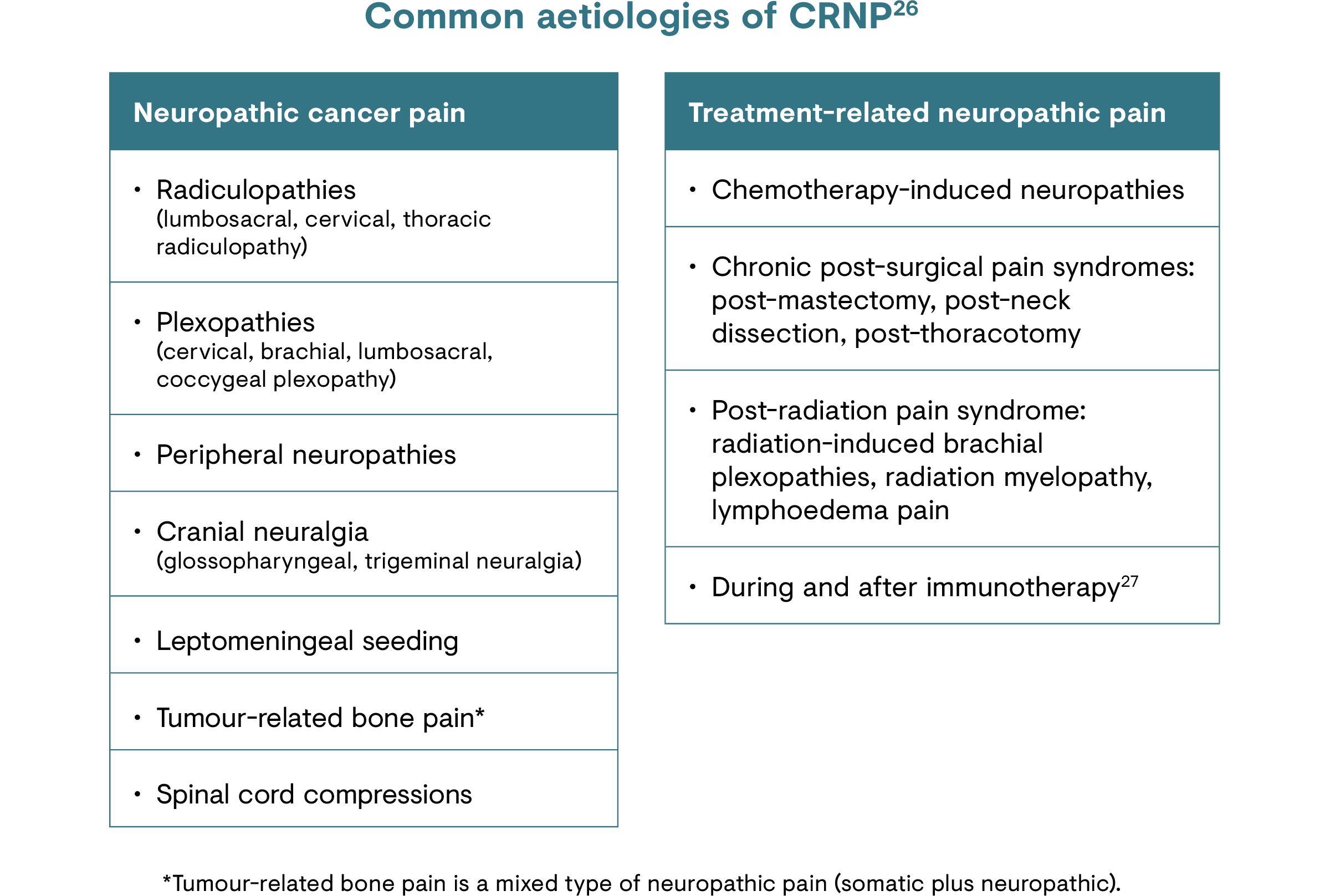
CRNP is caused by damage or injury to the peripheral or central nervous system, due to the primary tumour and/or its metastases or cancer treatments.24 The most common causes of cancer treatment-related neuropathic pain are chemotherapy, radiotherapy and surgical interventions.25
Common classes of chemotherapy drugs causing peripheral neuropathy include:1,23
- Platinum-based drugs (e.g. cisplatin and oxaliplatin)
- Taxanes (e.g. paclitaxel and docetaxel)
- Thalidomide and proteasome inhibitors (e.g. bortezomib)
- Vinca alkaloids (e.g. vincristine and vinblastine)
Signs and symptoms
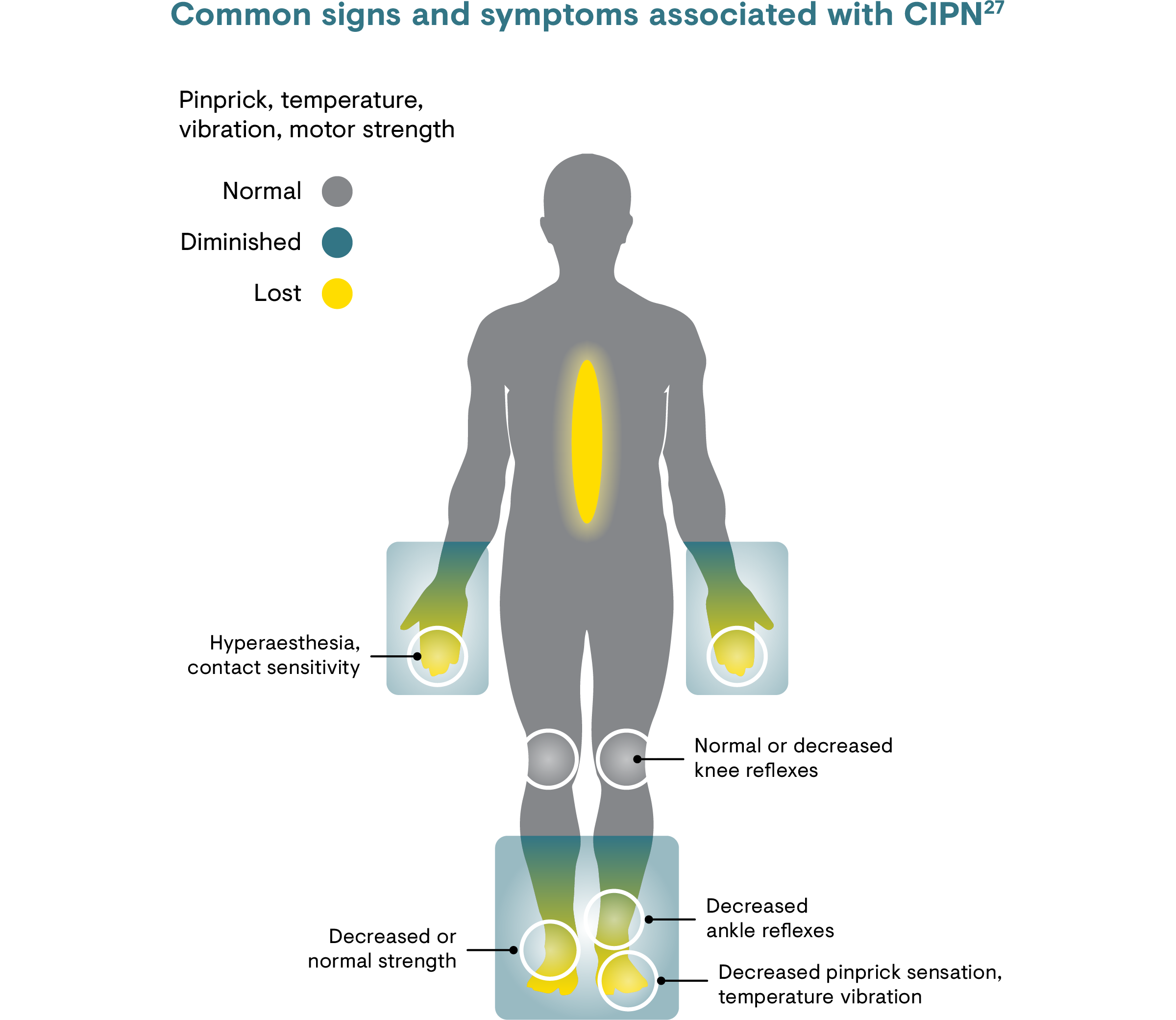
The signs and symptoms of PNP, including CRNP,8 include shooting pain, sharp pain, stabbing pain, tingling, pricking, electric shocks, and pins and needles.24 Specifically, painful CIPN can manifest in hands, feet and sometimes in the face, and can extend in a ‘glove and stocking’ distribution affecting lower arms and lower legs.1 Chemotherapy-induced neuropathy symptoms predominantly start as tingling and/or numbness that later develop into pain.23,27 The combination of CIPN symptoms may continue to develop and progress for several months after therapy (‘coasting effect’) and may persist for several months to years.28 The same late onset and persistence is also true for radiotherapy-induced neuropathic pain.25
Pathophysiology
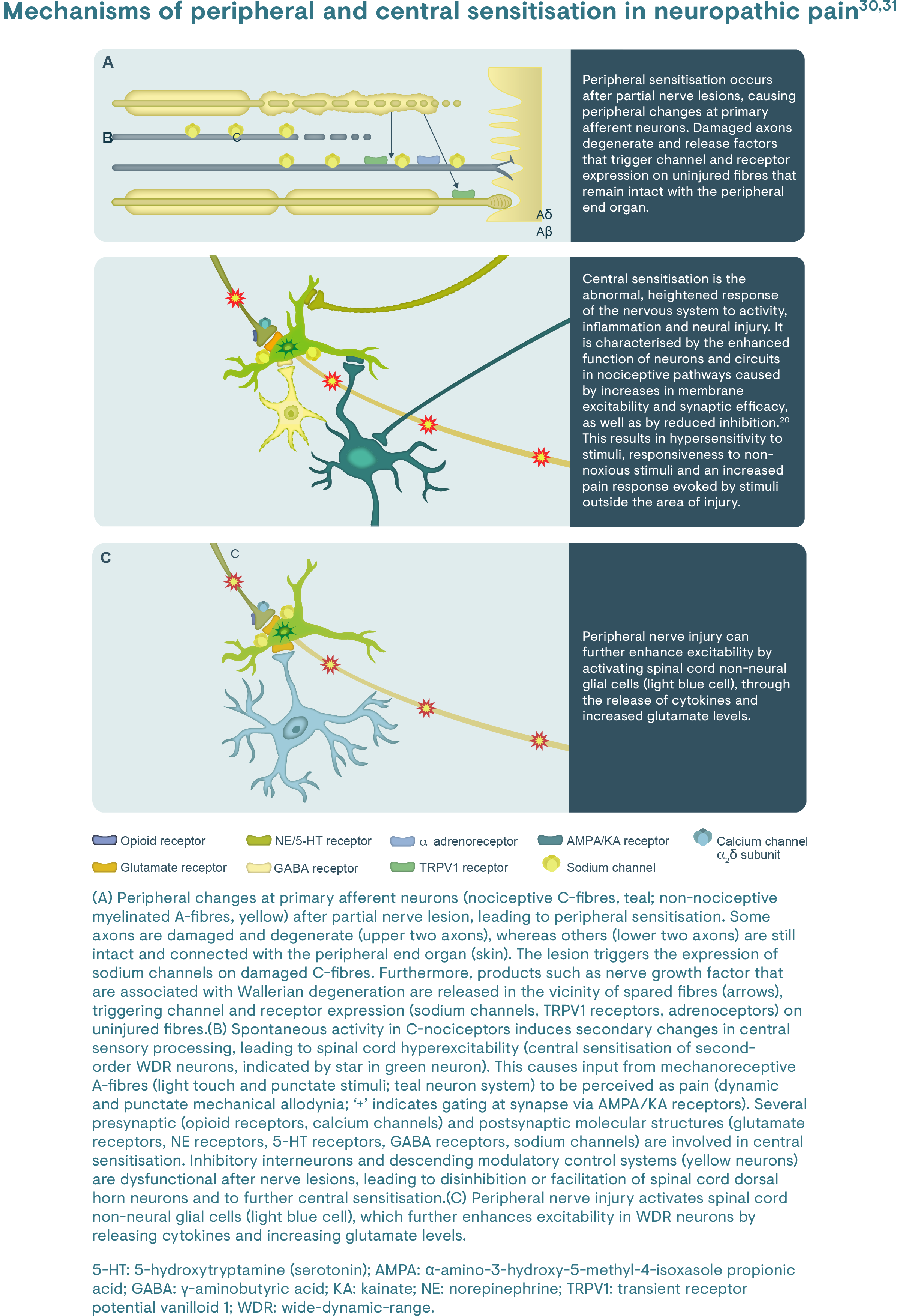
Pathophysiologically, CRNP occurs if the somatosensory nervous system is directly affected by tumour (primary or metastasis) infiltration or compression, tumour-associated toxins, therapy-related toxins or direct surgical damage.25 Damage to peripheral nerves results in changes at cellular and molecular levels that may cause sensitisation of the peripheral and central nerve pathways, similar to other PNP conditions [you can read more about the peripheral mechanisms here].29
CIPN is predominantly a sensory, axonal peripheral neuropathy that may be accompanied by motor and autonomic changes.23 Typically, longer axons are affected first, manifesting in the hands, feet and sometimes in the face.1,27
Diagnosis
CRNP can be difficult to identify,24 particularly if the pain begins months or years after treatment, as in the case of radiation therapy.25 However, it is important to identify whether the pain is caused by a (new) tumour, related to treatment or to other comorbidities.32 The diagnostic process of neuropathic pain in patients with cancer should be no different to that of non–cancer-related neuropathic pain.24
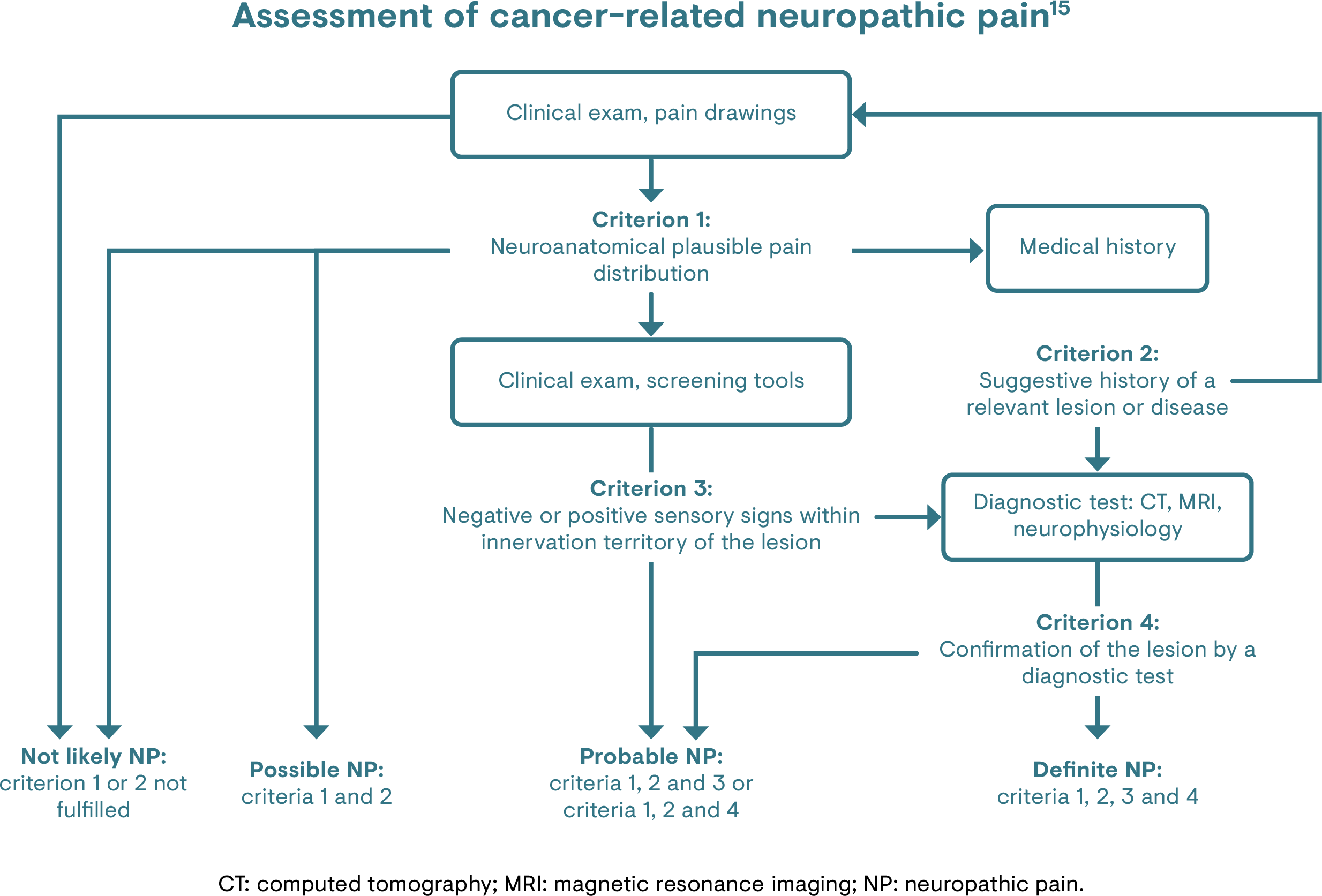
Probable or definite neuropathic pain can be identified using the revised definition and grading system proposed by the Neuropathic Pain Special Interest Group (NeuPSIG) of the International Association for the Study of Pain (IASP).33 Moreover, commonly used neuropathic pain-specific assessment tools, such as LANSS pain scale, DN4 questionnaire and PDQ have been evaluated for use specifically in CRNP.8–14
The assessment of a patient’s CRNP should be an ongoing process.15 The ESMO guidelines on the management of cancer pain discuss the assessment and reassessment of the pain (e.g. causes, pain quality descriptor) and the patient (e.g. clinical situation, functional status, presence of comorbidities).15 The guidelines also recommend that the assessment of all components of suffering, such as psychosocial distress, should be considered and evaluated.15
The International Classification of Diseases (ICD)-11 codes for the diagnosis of chronic cancer-related pain should be given in addition to the ICD-11 codes for the cancer itself. Cancer-related pain should be classified whether it was caused by the primary cancer itself, its metastases or its treatment.1
Management
Guidelines and recommendations
Neuropathic pain is associated with poor patient outcomes, and patients with cancer and neuropathic pain often report worse physical, cognitive and social functioning than those with cancer and nociceptive pain.9 Patients with CRNP have been shown to be associated with the need for more oncological treatment, a greater analgesic requirement and a reduced performance status, compared with those with nociceptive cancer pain.9
Healthcare professionals managing patients with CRNP should be aware that neuropathic pain may be aggravated by sleep disturbance, anxiety and depression.19 Non-pharmacological interventions are also important in the multimodal management of CRNP.24 Several systematic reviews suggest that mind–body interventions, such as acupuncture and mindfulness-based interventions, can offer a therapeutic benefit for improving chronic cancer pain.34
In terms of guidelines, the following organisations have published guidance on CRNP management:
- ESMO15
- EFIC17
- ASCO16
- ESMO–EONS–EANO19
- NCCN18

Pharmacological treatments
The goals of pain treatment are to reduce pain to a level that allows for a quality of life that is acceptable to the patient and to prevent the pain or medication adverse events leading to cancer treatment cessation.35
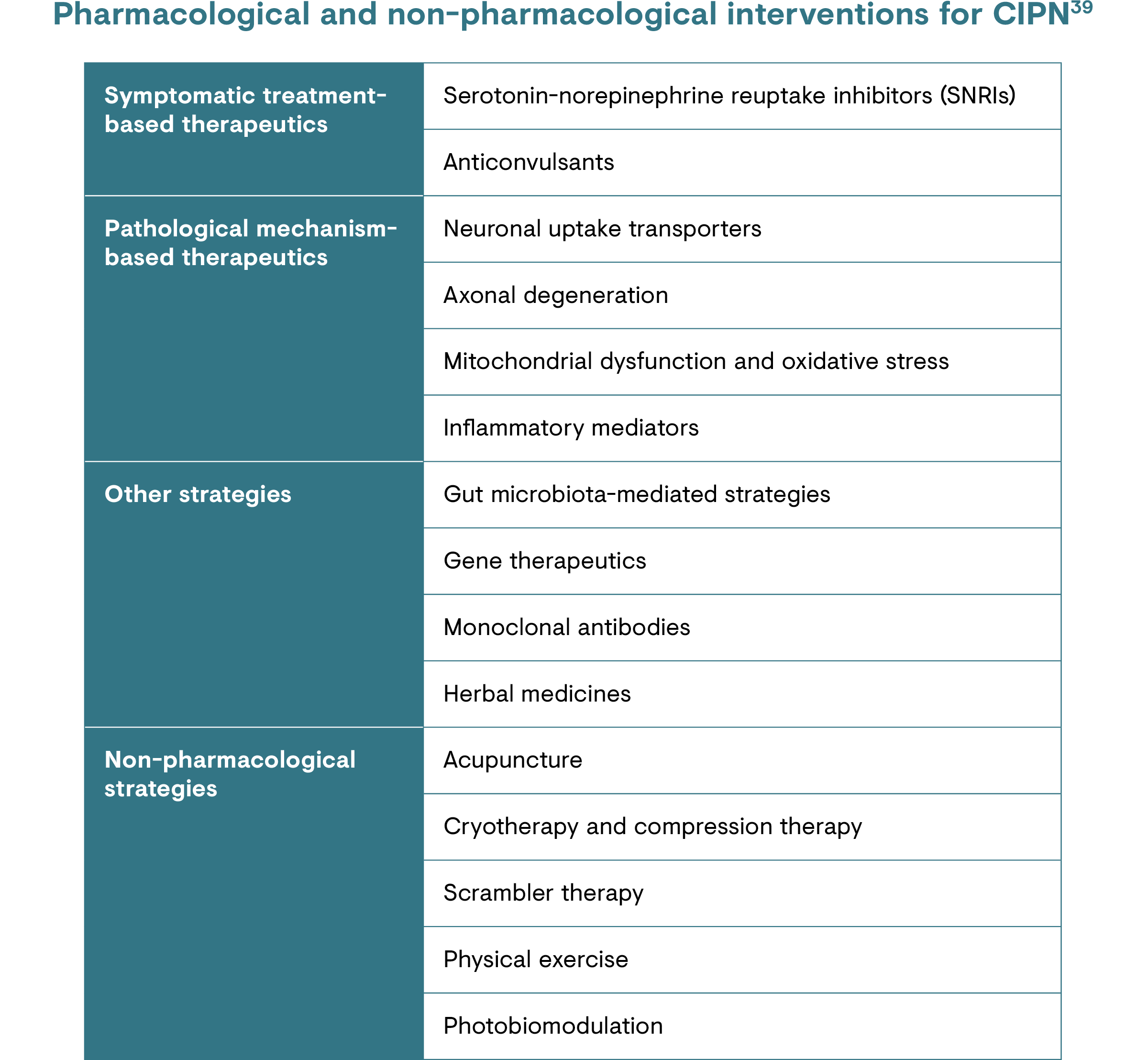
The treatments used in routine practice have barely changed since the World Health Organization published their approach 30 years ago.36 In addition, there is no clear consensus for first-line or stepwise treatment of CRNP.15,35 The recommended treatments within the ESMO guidelines for CRNP are a selection of those for peripheral neuropathic pain and are used because their mechanism of action is targeted to neuropathic pain.30,37
A mechanism-based approach to pain management in neuropathic pain, including in CRNP, considers pathophysiological and biological mechanisms, as well as behavioural and health systems processes, in order to prioritise effective interventions for patients. 38
Unmet needs
While our understanding of the complex neurobiology of pain has increased, pain assessment, cancer-related pain neurobiology and the use of targeted therapies require further research.24 From other pain conditions, we know that a thorough pain assessment can indicate pain mechanisms and guide targeted therapy; this, in turn, can improve patient outcomes.24 Therefore, it is important that the cause of CRNP is correctly identified in order to achieve optimal pain control.1
There is a need to refer cancer patients with neuropathic pain to specialist services as early as possible when their pain is not responding to initial therapy, rather than waiting until all conventional approaches have been exhausted and the patient is too unwell for advanced pain management intervention.15,17 Unfortunately, only a minority of cancer patients with pain are currently referred to a pain specialist, so may not be adequately treated.40
Inadequate patient education is also a key barrier to effect cancer-related pain management.41,42 Educational interventions and resources can support patients with their own self-management, including coping strategies, and have been shown to facilitate better cancer pain management.1,41
-
References
1. Bennett MI et al. Pain. 2019;160(1):38–44.
2. Scholz J et al. Pain 2019;160:53–9.
3. Lemiale V et al. Ann Intensive Care. 2019;9(1):25.
4. Seretny M et al. Pain 2014;155(12):2461–70.
5. Cavaletti G & Marmiroli P. Nat Rev Neurol. 2010;6(12):657–66.
6. Hershman DL et al. J Clin Oncol. 2014;32(18):1941–67.
7. Bennett MI et al. Pain. 2012;153(2):359–65.
8. Mulvey MR et al. Br J Anaesth. 2017;119(4):765–74.
9. Rayment C et al. Palliat Med. 2013;27(8):714–21.
10. Mercadante S et al. J Pain. 2009;10(6):594–600
11. Hardy J et al. Support Care Cancer. 2013;21(12):3387–91.
12. Bouhassira D et al. Pain. 2017;158(6):1118–25.
13. Pérez C et al. Eur J Pain (United Kingdom). 2015;19(6):752–61.
14. Potter J et al. J R Soc Med. 2003;96(8):379–83.
15. Fallon M et al. Ann Oncol. 2018;29(Suppl 4):iv166–91.
16. Loprinzi CL et al. J Clin Oncol. 2020;38(28):3325–48.
17. Bennett MI et al. Eur J Pain. 2019;23(4):660–68.
18. Swarm RA et al. Adult Cancer Pain. 2021. Available at: https://www.nccn.org/professionals/physician_gls/pdf/pain.pdf. Accessed June 2021.
19. Jordan B et al. Ann Oncol. 2020;31(10):1306–19.
20. Cancer Research UK. Worldwide cancer statistics. Available at: https://www.cancerresearchuk.org/health-professional/cancer-statistics/worldwide-cancer. Accessed June 2021.
21. Van Den Beuken-Van Everdingen MHJ et al. J Pain Symptom Manage. 2016;51(6):1070–90.e9.
22. Davis MP & Walsh D. Am J Hosp Palliat Med. 2004;21(2):137–42.
23. Starobova H & Vetter I. Front Mol Neurosci. 2017;10:174.
24. Edwards H et al. Cancer-Related Neuropathic Pain. Cancers (Basel). 2019;11(3):373.
25. Naleschinski D et al. IASP Pain Clin Updat. 2012.
26. Yoon SY & Oh J Korean J Intern Med. 2018;33(6):1058–69.
27. Kerckhove N et al. Front Pharmacol. 2017;8(FEB):86.
28. Argyriou AA et al. Crit Rev Oncol Hematol. 2012;82(1):51–77.
29. Gilron I et al. Mayo Clin Proc. 2015;90(4):532–45.
30. Baron R et al. Lancet Neurol. 2010;9:807–19.
31. Baron R. Nat Clin Pract Neurol. 2006;2:95–106.
32. Caraceni A & Shkodra M. Cancers. 2019;11(4).
33. Finnerup NB et al. Pain. 2016;157(8):1599–1606.
34. Eaton LH & Hulett JM. Semin Oncol Nurs. 2019;35(3):241–52.
35. World Health Organization. WHO Guidelines for the Pharmacological and Radiotherapeutic Management of Cancer Pain in Adults and Adolescents. 2018. Available at: https://apps.who.int/iris/bitstream/handle/10665/279700/9789241550390-eng.pdf. Accessed June 2021.
36. World Health Organization. Cancer Pain Relief: With a guide to opioid availability – 2nd Edition; 1996. Available at: https://apps.who.int/iris/handle/10665/37896. Accessed June 2021.
37. Bannister K et al. Annu Rev Pharmacol Toxicol. 2020;60:257–74.
38. Bennett MI et al. Pain. 2017;158(4):S74–S78.
39. Li Y et al. Cancers 2021;13(4):766.
40. Sancak Ö et al. In: 11th Congress of the European Pain Federation (EFIC). Valencia; 2019:P092.
41. Kwon JH. J Clin Oncol. 2014;32(16):1727–33.
42. Allsop MJ et al. BMJ Open. 2018;8(3):e021965.
